Sexually transmitted diseases in animal
systems
Sexually transmitted diseases, or STDs aren't just a problem
for people. They are widespread through the animal kingdom, being known to infect
a range of hosts as diverse as horses, geese, koala bears, ladybird beetles
and snails. What's more, they can cause considerable damage to their hosts,
often causing sterility, and yet we know hardly anything about them. In addition
to their possible importance as regulators of animal populations, STDs could
make important contributions to the evolution of mating systems, as they can
impose an obvious cost on mating, particularly in systems where a few males
mate with large numbers of females.
|
My research into STDs has followed two paths.
Firstly I have been involved in theoretical work, using mathematical models
to investigate the possible way in which STDs and their hosts might interact
in evolutionary terms, and secondly I have a laboratory based project
currently running to look at the way in which STDs are transmitted in
their host populations using an insect model.
Theoretical work. My research has looked at the possible
role of STDs in sexually selected systems from a theoretical point of
view (abstract here). I concluded
that some of the previous ideas about the possible role of STDs were likely
to be incorrect, since they didn't take into account the evolution of
the STD as well as that of the host.
More recently, I've been collaborating with Mike
Boots at the University of Stirling to develop a model that addresses
the apparent paradox that many animal species indulge in behaviour that
increases their risk of contracting an STD, such as mating with multiple
partners (abstract here). What we
found is that so long as there is even the tiniest fitness advantage to
multiple mating, the presence of a sterilising STD will tend to allow
coexistence of more and less promiscuous strains, rather than leading
to the elimination of the more promiscuous strain. |
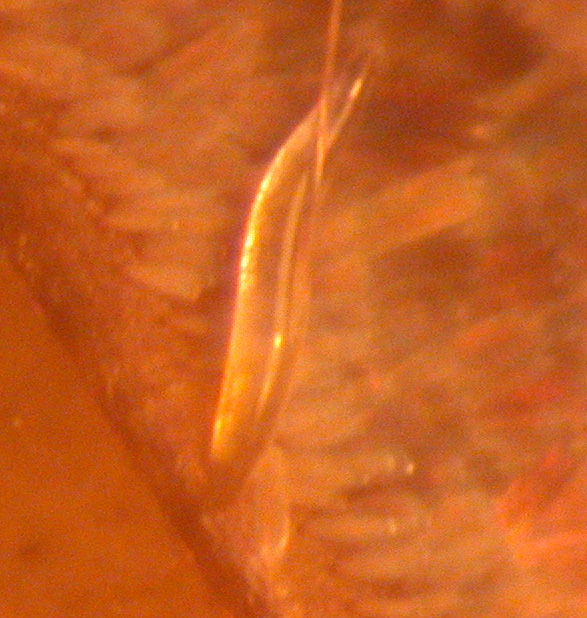
| This picture shows an STD of a moth. The STD is a nematode
worm called Noctuidonema guyanese which is widespread on noctuid
moths in Central and South America. It's feeding on the moth's haemolymph
(insect blood) through the membrane between the segments of the abdomen. |
Laboratory work. From a more ecological standpoint,
I currently have a large research project in progress to investigate the
relationship between the transmission rate of an STD (how many new infections
arise per infectious host per unit time) and the density of the host population.
Theoretical models of host-STD systems usually assume that transmission
rate does not change with host density, but is instead related to the
proportion of infectious hosts in the population. This has never been
experimentally tested and there are reasons to believe that real STDs
will show different transmission at different host densities.
This research is all funded by the Wellcome
Trust. People in my lab working on this project are Mary Webberley,
Jon Ryder and Hajir Al-Khairulla. |
 |
 |
We are carrying out experiments with the two-spot ladybird (Adalia
bipunctata) as a host and a sexually transmitted mite parasite (Coccipolipus
hippodamiae) as the STD. The use of an insect system means that it
is possible to do very detailed experiments that would not be possible
with a vertebrate system. The laboratory work will be used to guide the
development of mathematical models to give us information on how changes
in transmission dynamics can affect the development of STD epidemics,
and how best to control them.
The sexually transmitted mite parasite was originally described as such
by Greg Hurst from UCL and his co-workers. It is not found in British
ladybirds but can reach very high prevalences in continental populations.
The mite lives on the underside of the elytra (the wing cases) of the
beetle. Adult mites suck haemolymph (insect blood) from the host, and
lay eggs that hatch into mobile larvae. When the ladybird mates these
larvae walk across from one host to the other, and then develop into adults
on their new hosts. Almost all transmission of the mite occurs during
mating. Female ladybirds that are infected become sterile after a few
weeks.
|
| This picture shows an adult and some larvae of Coccipolipus
hippodamiae on the underside of a wing case from a two-spot ladybird.The
adult is the large orange object with the white stripe, the larvae are the
smaller paler blobs with legs. |
If you want to know more, the project
description from the grant appplication is here.
This link connects to an article that was in Wellcome
News about the project. It features a particularly unflattering photo of
me.
This takes you to the website for 'The
Material World', a BBC Radio 4 show which had a feature on this project.
If you're really bored you can listen to it in streaming audio...
Here are a few references if you'd like to get into the literature a bit more
The ladybird-STD system
Hurst GDD, Sharpe RG, Broomfield AH, Walker LE, Majerus TMO, Zakharov IA, Majerus
MEN (1995) Sexually transmitted disease in a promiscuous insect, Adalia bipunctata.
Ecological Entomology 20: 230-236
Webberley, KM, Hurst, GDD, Buszko J, Majerus MEN (2002) Lack of parasite mediated
sexual selection in an invertebrate-STD system Animal Behaviour 63: 131-141
General information about animal STDs
Lockhart AB, Thrall PH, Antonovics J (1996) Sexually transmitted diseases in
animals: ecological and evolutionary implications. Biological Reviews. 71: 415-471
Theoretical stuff about transmission dynamics
Getz WM, Pickering J (1983) Epidemic models: thresholds and population regulation.
American Naturalist 121: 892-898
Hamilton WD (1990) Mate choice near or far. American Zoologist 30: 341-352
McCallum H, Barlow N, Hone J (2001) How should pathogen transmission be modeled?
Trends in Ecology and Evolution 16: 295-300
Canfield, PJ, Brown AS, Dickens RK (1991) Disease status of the koala (Phascolarctos
cinereus) Avian/Exotic Practice 3: 21-26
Thrall PH, Antonovics J, Hall DW (1993) Host and pathogen coexistence in sexually
transmitted and vector-borne diseases characterised by frequency-dependent disease
transmission. American Naturalist 142: 543-552
Thrall PH, Biere A, Uyenoyama MK (1995) Frequency dependent disease transmission
and the dynamics of the Silene-Ustilago host-pathogen system. American Naturalist
145: 43-62
Thrall PH Antonovics J, Bever JD (1997) Sexual transmission of disease and host
mating systems: within season reproductive success. American Naturalist 149:
485-506
Back to my home page

